Book Your Free Demo Now!
Top 50 Questions of Carbon and its Compound Lesson
Question: 1. Name one of the triple bond
C3H4
Question: 2. Write ethane with bond name
C2H4 and seven bond
Question: 3. Vinegar has a smell of...
Acetic Acid
Question: 4. Another name of ethanoic acid
Acetic Acid
Question: 5. Which gas does LPG consists
Butane
Question: 6. Hard water contains
Ca2+ and mg2+
Question: 7. Write the boiling point of ethanol
78 degree C
Question: 8. What is formed in the esterification process?
Easter
Question: 9. What is used as syringes in hospitals and antiseptic for wounds?
Ethyl alcohol
Question: 10. Draw any two dot structures with their names.
Methane structure and Hydrogen fluoride
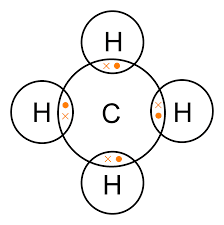
Question: 11. Write the structural formula of the isomers of hexane.
CHC3(CH2)4CH3
Question: 12. Draw an ester diagram.
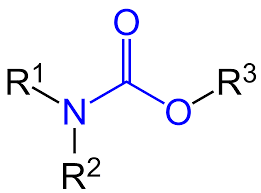
Question: 13. What is the hydrogenation reaction?
Reaction with hydrogen.
Question: 14. What did you understand by oxidation reaction?
Reaction with oxygen
Question: 15. What is a Substitution reaction?
When one kind or group of atoms replace some other atom.
Question: 16. What is meant by a saturated hydrocarbon?
That Hydrocarbons in which the valency of carbon is satisfied by single bonds
Question: 17. Describe the structure of a CH3COOH molecule.
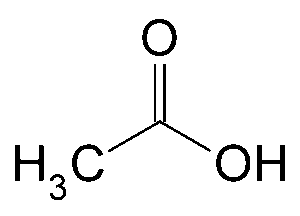
Question: 18. Describe the structure of an ethanol molecule.
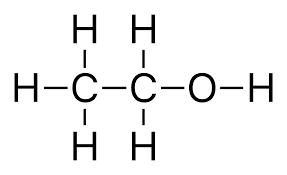
Question: 19. Butanone is a four carbon per molecule compound. Name the functional group present in it.
Ketone
Question: 20. Name the functional group present in each of the C2H5Cl and C2H5OH and HCOOH
They are following:
- (-Cl) Halogen (Chloro)
- (-OH) Alcohol
- (-COOH) carboxylic acid
Question: 21. Write the functional group name of -CHO.
Methanal HCHO
Question: 22. Write the functional group name of HCOOH.
Methanoic acid
Question: 23. What does soaps contain?
A long chain of fatty acids with sodium or potassium salt. It's RCOONa or RCOOR as A molecular formula.
Question: 24. What do you understand by heteroatoms?
The other atoms than C and H bonds are to be heteroatoms.
Unlock Expert Guidance – Book Help Now!
Avail now by providing the below details
Error: Contact form not found.

Question: 25. Why do covalent compounds have low melting and boiling points?
The binding molecules have weak force and need less energy to break the force of Bonding therefore.
Question: 26. Diamond and graphite show different physical properties although they are made up of carbon and shows the same chemical properties. What is the property called?
Isotope- similar in proton number but differential in neutron number.
Question: 27. Define catalyst.
Those atoms which are added to a reaction to speed up chemical reaction without losing spare Energy and without disturbing the structure are known as catalysts.
Question: 28. What are the chemical properties of carbon compounds?
Below mentioned are the properties:
- Combustion reaction
- Substitution reaction
- Substitution reaction
Question: 29. Write an additional reaction?
When unsaturated hydrocarbon combines with another substance to make a new compound.
Question: 30. Write some uses of ethanol.
Uses of ethanol are:
- Manufacturing paints
- Cough syrup
- Alcoholic drinks etc
Question: 31. How is ethene prepared from ethanol?
With the help of dehydration method when sulphuric acid concentrates within the reaction of ethanol into ethene
Question: 32. Intake of methanol even a small quantity can be lethal Comment.
Methanol and formaldehyde is toxic and carcinogenic and even causes blindness
Question: 33. What do you understand by catenation?
It is a process of bonding of two similar atoms in different conditions that can only happen in carbon and silicon.
Question: 34. What is the Saponification reaction?
The method of forming soaps, in which potassium and sodium salts of long chains fatty acid through which esters with organic base react with each other and forms alcohol and soap.
Question: 35. What would be the reaction of propanol to propanoic acid?
When propanol reacts to propanoic acid in the presence of sulfuric acid to give propyl propanoate an ester compound.
Question: 36. What is denatured alcohol?
Alcohol is consumption but not denatured means adding one or more chemicals to it to making alcohol so not to drink is known as denatured
Question:37. A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Hydrogen gas evolved and reaction between ethanol with sodium gives sodium ethoxide is C2H5O-Na+
Question: 38. Explain isomerism. State any four characteristics of isomers.
Those element or compound having similar molecular formula but different structural formula are called isomerism.
Characteristics of isomers are:
A. They differ in structural formula,
B. They differ in melting point,
C. They differ in boiling point,
D. They differ in solubility in the same solvent.
Question: 39. Difference between esterification and saponification reactions of organic compounds with the help of the chemical equation for each. What is the use of esters and the saponification process.
Esterification: Ester is formed when alcohol reacts with carboxylic acid within H2SO4 concentration presence.
Saponification: When an ester reacts with acid to make alcohol
Uses of saponification and esterification:
(i) Esters are used in cold drinks,
(ii) Ice cream
(iii) Perfumes and
(iv) As artificial flavouring agents(v) Saponification process is used in the manufacture of soaps.
Question: 40. List two merits and demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing, even in the case of water having calcium and magnesium ions.
Merits:
1. Easily soluble in hard water.
2. More mixable than soap.
Demerits:
1. Expensive in price
2. Non-biodegradable, that is why water pollution
Detergents are suitable for hard water having Mg2+ and Ca2+ ions.
Question: 41. What is homologous series of compounds? List any four characteristics of homologous series.
A group of particular compound with the same functional group of Elements properties
Characterstic of homologous series are:(i) Common formula
(ii)Common difference of -CO2
(iii) Different in molecular mass
(iv) Similar chemical qualities
Question: 42. Explain the cleansing action of soap.
Actions of soap
1. Soap contains hydrophobic part which anions are soluble n grease and water
2. Reduces the surface tension of water
3. Wet the clothes with enough water
4. Rub or scrub the cloth to pull grease away from the spot and broken into many smaller droplets
5. Droplets mixed with water forming lather or emulsion
6. Red position of droplet on the cloth and rinse with water to away the droplets
Question: 43. An organic compound A on heating with concentrated H2SO4 forms a compound B which in addition to one mole of hydrogen in presence of Ni forms a compound C. One mole of compound C on combustion forms two moles of CO3 and 3 moles of H2O. Identify the compounds A, B and C and write the chemical equation of the reaction involved.
Reactions:
A. C2H5OH ----------> H2C = CH2
B. CH3 - CH3 +7/2O2 -------> 2CO2 + 3H2O
C. CH 2 = CH2 + H2----------> CH 3 - CH3
In which compounds are
● A= Ethanol
● B= Ethene
● C= Ethane
Question: 44. A compound C (molecular formula, C2H4O2) reacts with Na - metal to form a compound R and evolves a gas which burns with a pop sound. Compound C on treatment with an alcohol A in presence of an acid forms a sweet smelling compound S (molecular formula, C3H6O2).In addition of NaOH to C, it also gives R and water. SON treatment with NaOH solution gives back R and A. Identify C, R, A, S and write down the reaction involved.
Reaction processes
A. 2CH3COOH + 2Na --------> 2CH3COONa + H2
B. 2CH3COOH + CH3OH ------------> CH 3COONa + H2O
C. 2CH3COOH + CH3OH --------------> CH 3COOCH + H2O
D. CH3COOCH3 +NaOH -------------> CH 3COONa + CH3OH In which
● R =Sodium acetate ( sodium salt of ethanoic acid)
● C = Ethanoic acid
● A = Methanol
● S =Ester (methyl acetate)
Question: 45. A compound X is formed by the reaction of a carboxylic acid C2H4O4 and an alcohol presence of a few drops of H2SO4. The alcohol on oxidation with alkaline kMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the name of structures of 1. carboxylic acid 2. alcohol and 3. the compound X. Also write the reaction.
When acid and alcohol react, it becomes an esterification reaction. Given the alcohol upon oxidation with alkaline KMnO4 gives C 2H4O4,
Means the alcohol is ethanol and carboxylic acid is ethanoic acid which gives the ethyl ethanoate.CH3COOH + CH3CH2OH ------>
CH3COOCH2CH3( it is value of X)
Question: 46. Why are detergents better in cleaning qualities more than any soap? Explain
Points to be noted
1. Calcium and magnesium does not form lather in hard water
2. More soluble in water
3. Not making scum on clothes.
Question: 47.Differentiate between saturated and unsaturated hydrocarbons.
| Saturated Hydrocarbon | Unsaturated hydrocarbon |
|---|---|
| single bonds | double and triple bond |
| Burns with a blue, non-sooty flame | Burns with yellow sooty flame |
| Low solubility in water | Low solubility in water and can dissolve in nonpolar organic solvents. |
| Obtained from fossilized plant and animal materials. | From plants (pigment, waxes, proteins etc.) |
| Less reactive | Addition reaction property |
| eg: Methane , Ethane. Hexane, Butane, Pentane | eg:Ethene, Benzene, Propene, Butadiene, Acetic acid etc. |
Question: 48. Describe esterification and its uses?
Reaction of alcohol and acid with each other formed ester as the reaction product. Carboxylic acid (such as hydrochloric acid and sulfuric
acid) esters are like RCOOH within the reaction with alcohol , that is why this process is called esterification.
Uses of esterification are
(i) Fragrant odours such as in essential oils food flavouring cosmetics and in perfumes.
(ii) Naturally in pheromones.
(iii) Organic solvent
(iv) Fatty acid esters of glycerol is occurring naturally in fats and oils.
(v) Polyesters (in the process of making clothing).
(vi) Also used in soaps and detergent
Question: 49. Differentiate between soap and detergent.
| Soap | Detergent |
|---|---|
| Made up of natural ingredient | Made up of synthetic ingredient |
| Biodegradable | Non- biodegradable |
| Made from animal and plant fat and alkaline specification method | Are carbonic compounds which are not alkaline means from petrochemicals |
| Are sodium or potassium salts | Are ammonium or sulfonate salts |
Question: 50. Write some properties of ethanol?
Following are.
(i)CO 2 and H2O would release by burning in air.
(ii)Liberation of H2 during the reaction with sodium
(iii)Ethene got converted during H2SO4 present.
Like? Share it with your friends
-
Facebook
-
Twitter
-
Linkedin
-
Whatsapp
-
Reddit
Recent Posts
How to Prepare for an Exam: Key
Struggling with Reading? How to Boost Your
Online Tuition for Class 5 CBSE –
Balancing Screen Time and Study Time: A